Hybridization of SO2 (Sulphur Dioxide) - BYJUS In sulphur dioxide, the hybridization that takes place is sp 2 type. To determine this, we will first look at the sulphur atom which will be the central atom. During the formation of SO 2, this central atom is bonded with two oxygen atoms and their structure can be represented as O=S=O. SO2 Lewis Structure, Hybridization, Molecular Geometry, and ... 1 day ago · The hybridization of SO2 is Sp2. Now hybridization of SO2 can be understood in two ways, one is the theory and the 2nd is directly applying the formula. I would suggest learning the theory first and then you can surely go for the formal.
Hybridization of sulfur in sulfur dioxide May 14, 2016 · As seen, all the atoms have s p X 2 hybridization. I'll only focus on the central sulfur atom. Two s p X 2 orbitals form σ -bonds with the two oxygens. The other s p X 2 orbital is where the lone pair lives in. Now, we have dealt with 4 electrons, and only have 2 electrons to deal with.
Hybridization of so2

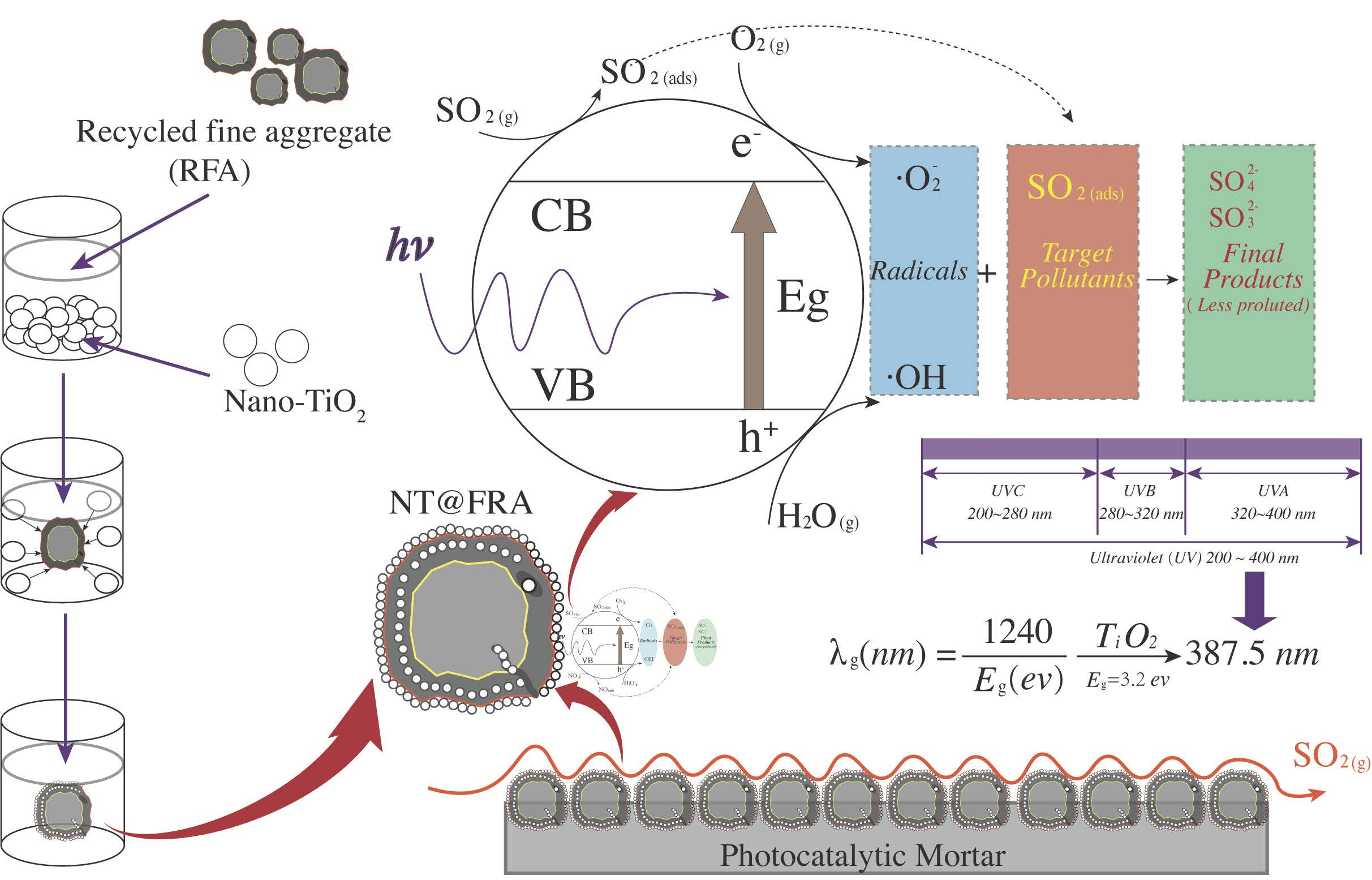
Nanomaterials | Free Full-Text | Sulfur Dioxide Degradation ...

What is the hybridization of the sulfur atom, electronic ...
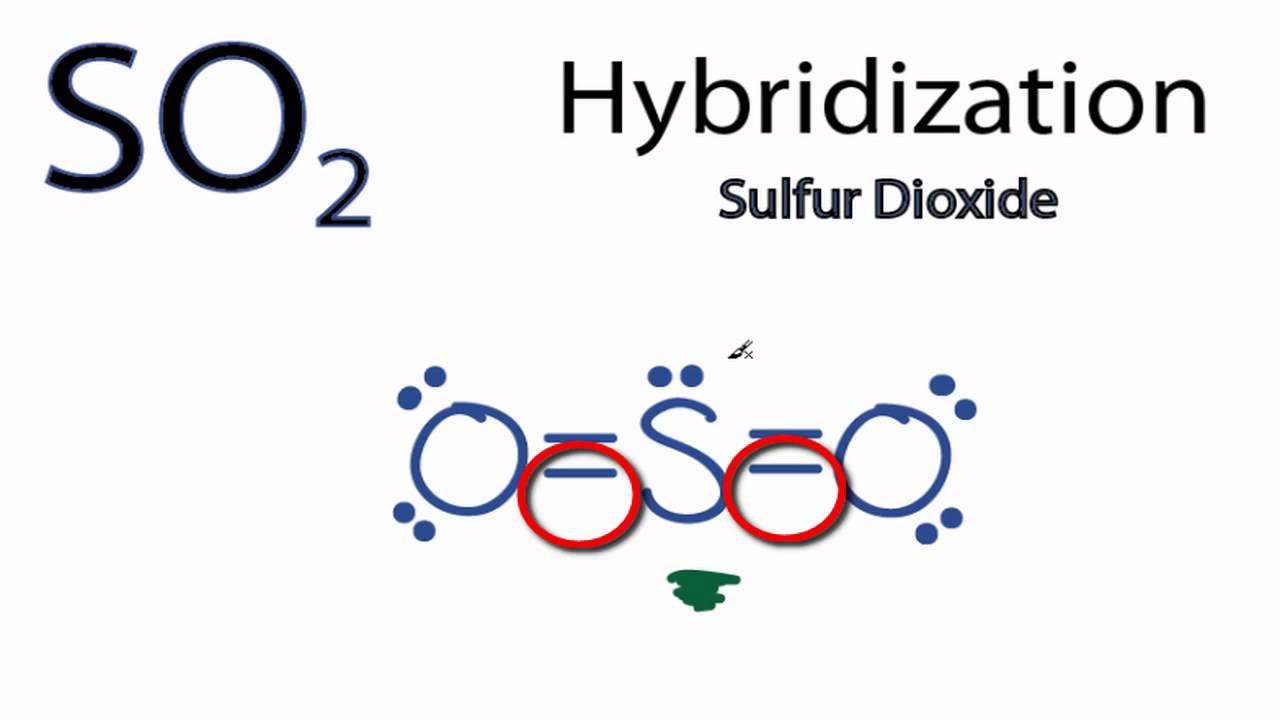
SO2 Hybridization
Hybridization: Definition, types and examples - Chemistry Notes

Hybridization

Explain the hybridisation in so2 - Chemistry - Chemical ...
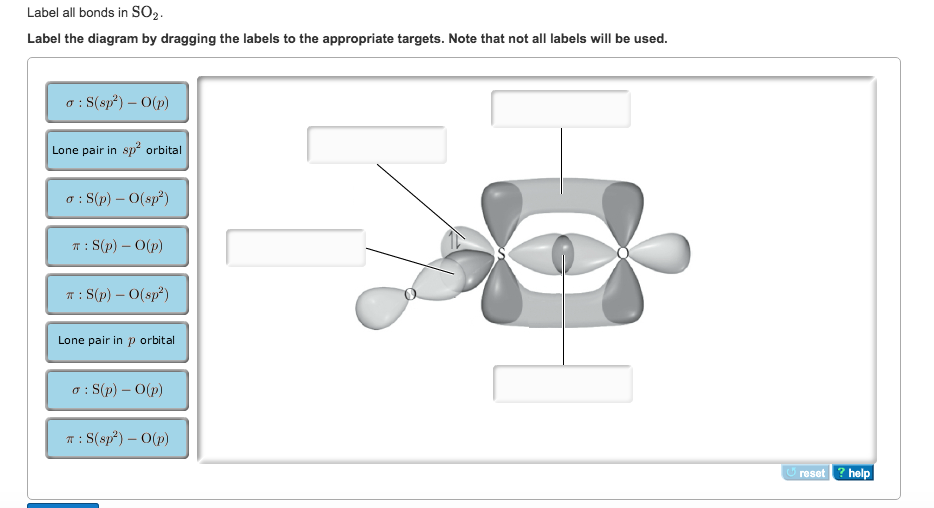
Solved Label all bonds in SO2. The hybridization of the S ...
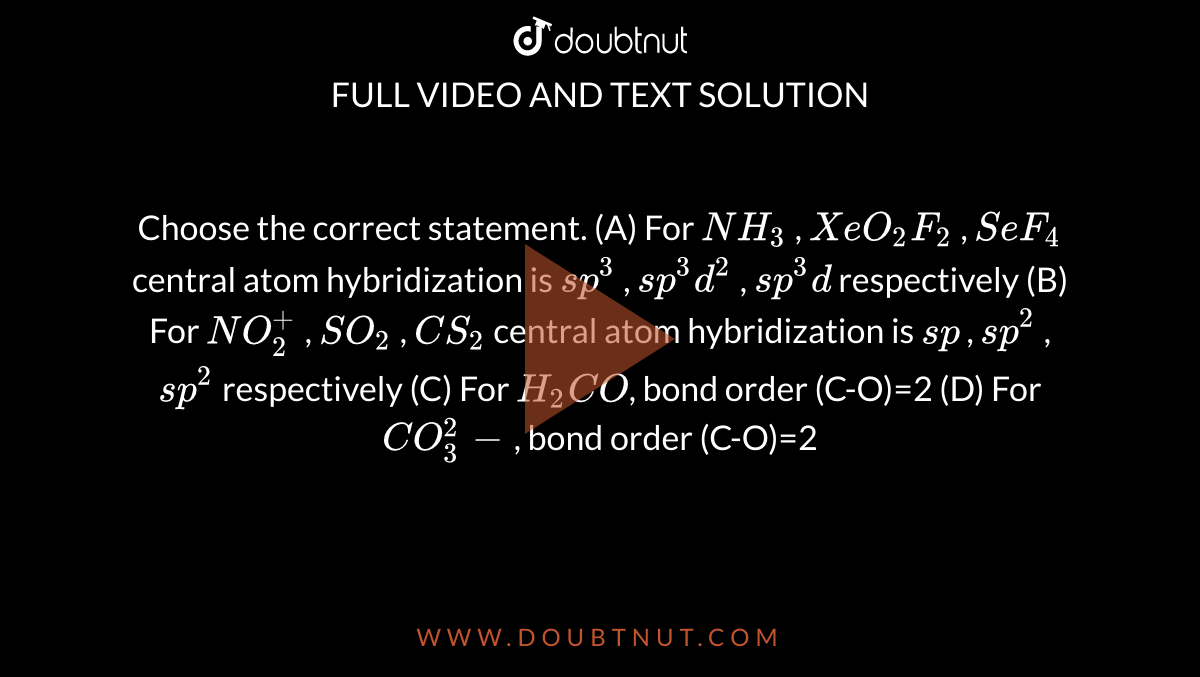
Choose the correct statement. (A) For NH3 , XeO2F2 , SeF4 ...

Why is the bond order in the SO₃ molecule 1.33 and not 2 ...
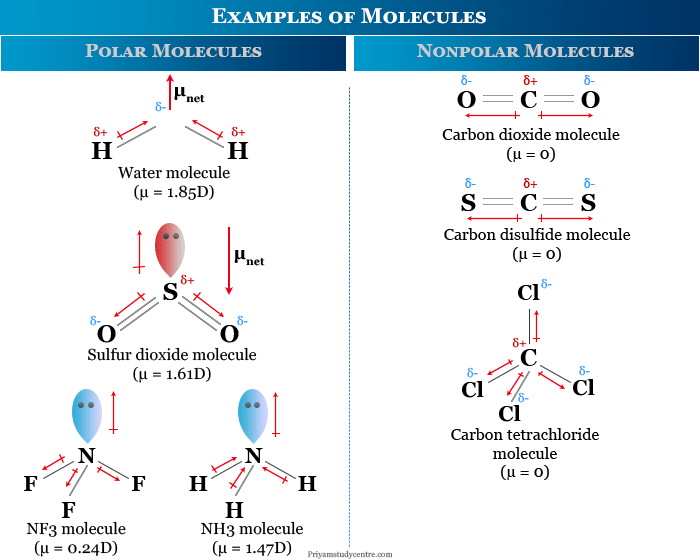
Molecule - Definition, Examples, Structure, Hybridization

SO2 Formal charge, How to calculate it with images?

SOLUTION: Chemistry bsc topic hybridisation hybridization of ...

The hybridizations of central atom in SO2 and CO2 are ...
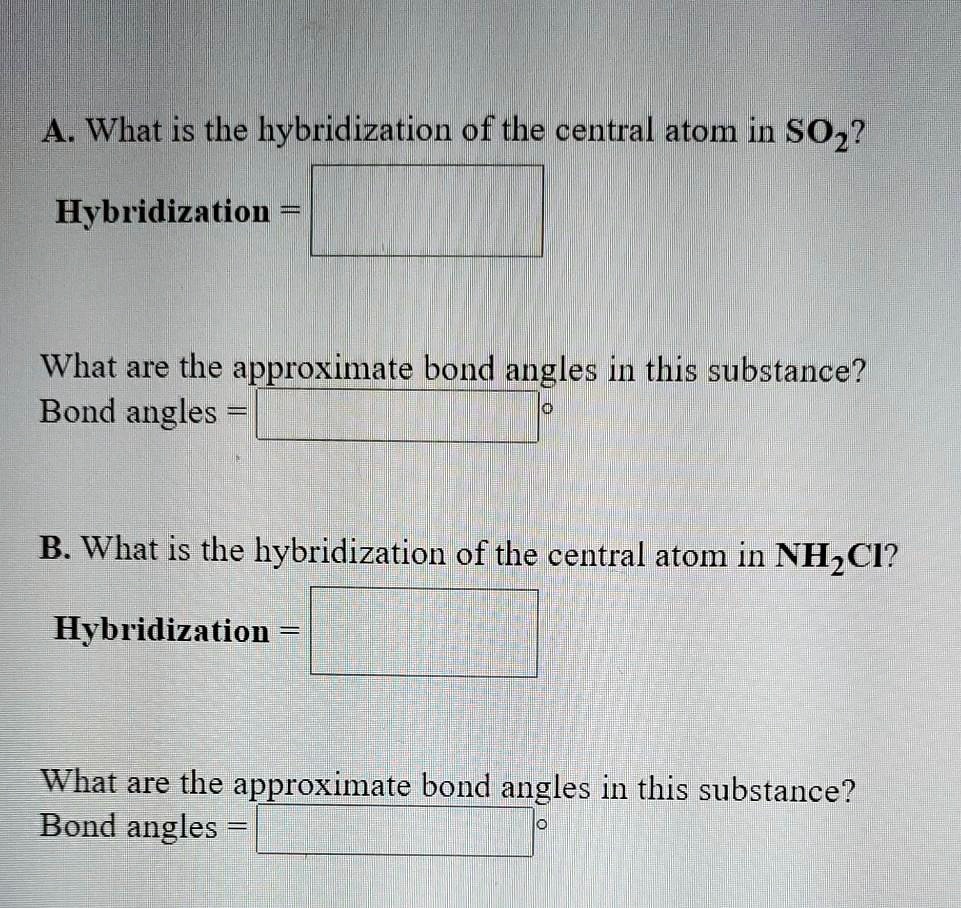
SOLVED: AWhat is the| hybridization of the central atom in ...

Sulfur dioxide (SO2) Lewis Structure, Hybridization
![Solved] Answer the following questions: Draw a lew structure ...](https://www.coursehero.com/qa/attachment/13805952/)
Solved] Answer the following questions: Draw a lew structure ...

Sp2 Hybridisation, VSEPR, Bent Geometry, SO2, Example - Chemistry

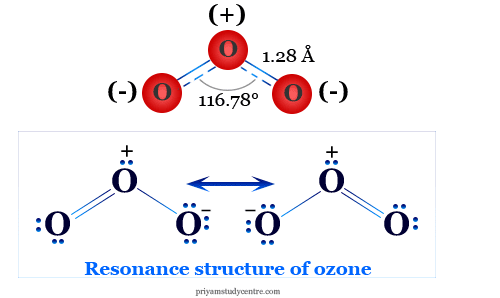
SO2 Lewis structure, Molecular geometry, Bond angle, Shape
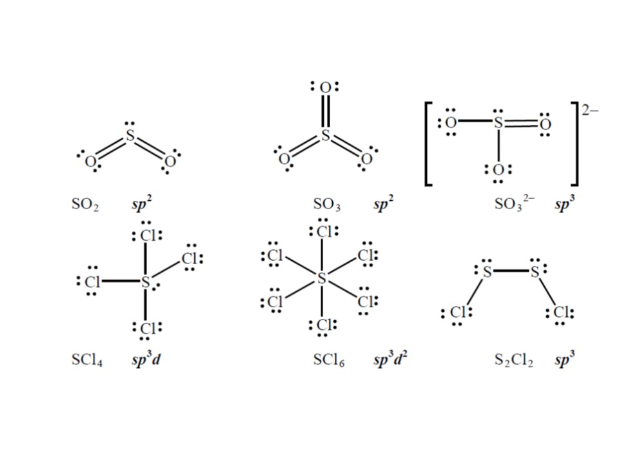
Solved Sulfur forms oxides, oxoanions, and halides. Draw ...

Answered: b. Identify the molecular geometry… | bartleby
Molecule - Definition, Examples, Structure, Hybridization

Explain the Structure of Sulphur Dioxide. - Chemistry ...

Sulfur dioxide (SO2) with hybridisation - 3D model by ...

What is the hybridisation of sulphur dioxide? - Quora

How is the hybridization of SO3 determined? - Quora
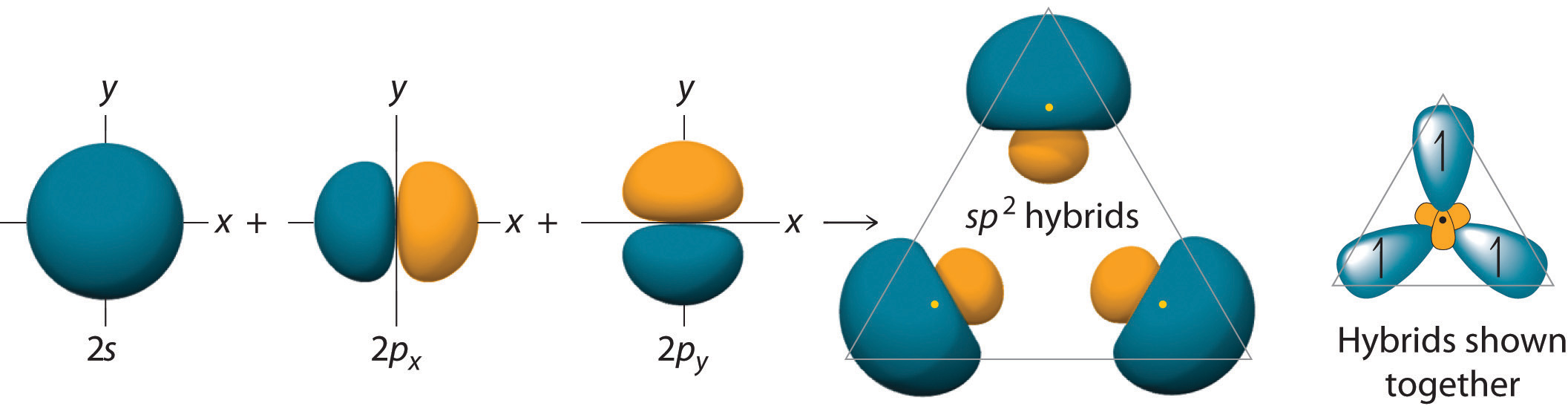
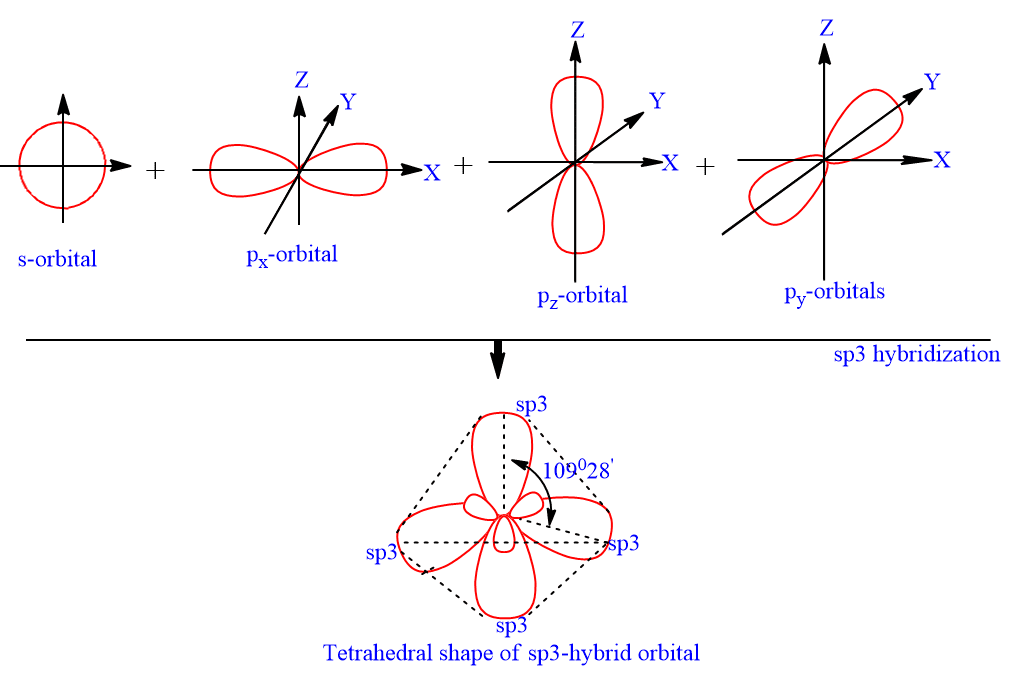
11.3: Hybridization of Atomic Orbitals - Chemistry LibreTexts

SOLUTION: Chemistry bsc topic hybridisation hybridization of ...

Sulfur dioxide (SO2) Lewis Structure, Hybridization
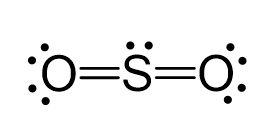
SO2 Geometry and Hybridization - Chemistry Steps

SO2 Lewis Structure, Hybridization, Molecular Geometry, and ...

SO2 Lewis Structure| 4 Simple Steps – What's Insight

The hybridization of S atom in SO2 is | Filo

Latest Info on SO2 Molecular Geometry - Education Is Around

SO2 Lewis structure, molecular geometry or shape, electron ...

Sulfur Dioxide | Umicore

Describe the bonding in SO2 and SO3 using the localized ...

The hybridization of orbitals of N atom in NO3^-, NO2^+ and ...
Valence Bond Theory & Hybridization: Chemical Bonding Class 11 Chemistry (Ch-4) | JEE Main 2022 Exam

Hybridization Examples in Chemistry|Types|sp|sp2|sp3|sp3d ...

What is the hybridisation of SO2? - Quora

shape of SO2 in hybridization - Brainly.in

SO2 Lewis structure, Molecular geometry, Bond angle, Shape























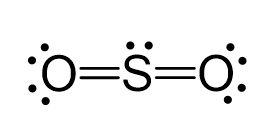










Komentar
Posting Komentar